Category Archive for "Pharmaceutical sector"

Barometer Study: Pharmaceutical Sector in Georgia
The study includes the data of the first two quarters of 2023, namely the indicators of the level of inflation on the health care group, and in particular on pharmaceutical products.

NEW Barometer, study focusing on the pharmaceutical sector
The Curatio International Foundation publishes a new Barometer, a study is focusing on the
pharmaceutical sector. The purpose of the study is to examine the inflation on healthcare
with a focus on pharmaceuticals.

Promote evidence-based policies in the pharmaceutical sector by generating evidence
The project will achieve the aim through two objectives: Investigate the recent reforms in the pharmaceutical sector and their impact on the access to medicines for Georgia citizens.
Promote civic engagement and enhance the role of civic society in advocacy towards sustainable policy solutions in access to affordable medicines.

External Reference Pricing Policy: A Possible Pharmaceutical Price Regulation Policy in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
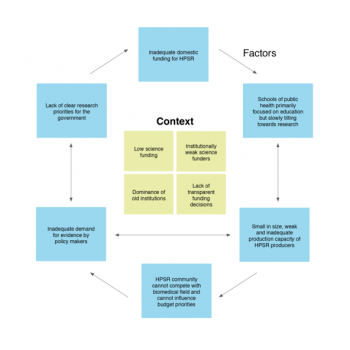
Paper: Soviet legacy is still pervasive in health policy and systems research in the post-Soviet states

Big Pharma Greed and Artificial Prices – Knocking on Door to Limit Access to HIV Medicines in Georgia
They produce medicines that can save lives and at the same time artificially restrict access to these drugs for financially deprived people and for countries that fall outside their commercial interests.
CIF Pharmaceutical Price and Availability Study in Georgia
Status: Closed project Introduction and Overview The goal of this project is to generate evidence and information on pharmaceutical prices and their availability at pharmacies; the enforcement of regulations regarding prescriptions; as well as pharmaceutical industry practices, in order to…

CIF Pharmaceutical Price and Availability Study (Fifth Wave Results)
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the…
Rapid Assessment of Pharmaceutical Sector and Drug Supply Chain
Rapid Assessment of Pharmaceutical Sector in Uzbekistan project was funded by The World Bank and implemented by Curatio International Foundation in partnership with Credes (France). The project commenced in 2003. The main objective of the project was to perform a…
Fourth Wave Results of Pharmaceutical Study Published
Curatio International Foundation has completed a study exploring ‘Price, Affordability and Availability of Medicines in Georgia’. The study was divided into three stages and carried out in 2009-2001. The key aim of the study is to improve affordability and availability…
