CIF Pharmaceutical Price and Availability Study (Fifth Wave Results)
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the continuous monitoring of the prices of medicine. One of the chief aims of the study is to inform and strengthen health policy and contribute to evidence-based discussions around current trends and processes in pharmaceutical market in Georgia.
The study analyzes the current, as well as the previous four waves of the PPA studies that have been conducted by CIF since 2009. The main findings of the research responds to two important questions:
- What is the trend of physical and financial availability for Generic (LPG) and Originator rand (OB) drugs in Georgia, and how is the treatment cost linked to the availability?
- How is Georgia’s pharmaceutical sector being developed after the introduction of the new prescription policy?
The answers to these questions are available in the main findings of the study:
Main Findings
AVAILABILITY
- Currently, OB availability is almost two-times higher compared to LPG
- The observed trend in decreased LPG availability can be attributed to several factors:
-
- Insufficient knowledge and/or trust in the quality of LPGs among consumers and providers
- Low demand for LPGs among the population caused by physician reluctance to prescribe generic medicines
- The revenue-maximizing strategy of pharmaceutical suppliers

TRENDS IN PRICING STRATEGIES
- It is likely that increased competition caused by legal changes in the country’s drug laws in late 2009 determined the downward trend in the OB prices observed during 2009-2012 waves, albeit OB prices rebounded and significantly increased in 2016
- OBs are largely imported from western countries. Therefore, it is possible that the price increase documented in 2016 can be partially attributed to the significant devaluation of the country’s national currency against the USD and Euro that began in late 2014, and continued throughout 2015. Consequently, OB prices increased in both pharmacy networks and in independent pharmacies
- In 2015, MoLHSA initiated a new prescription policy with the aim of reducing the level of irrational drug use in the Country. The importance and/or need for prescription system introduction is unquestionable, like in many other countries; however, the insufficiency and/or lack of the necessary instruments for the effective operation of the system most likely allowed pharmaceutical companies to use this initiative to further increase their profits. This assumption is supported by the fact that while in 2012 markups were largely comparable for prescription and non-prescription drugs, in 2016, we observed significant changes in behavior. Namely, markups for prescription OBs are now 89% higher compared to non-prescription OBs, and markups on prescription LPGs are currently 210% higher compared to non-prescription LPGs
- Surprisingly, locally-manufactured LPGs are sold at a higher price compared to their imported equivalents, most likely affording greater profit potential to local manufacturers. Along with the marketing strategies used by the largest retail networks (also linked to local manufacturing), the promotion of locally-produced drugs over imported drugs helps local producers effectively use their market power in a poorly-regulated marketplace
EMERGING POLICY RECOMMENDATIONS
A single policy intervention in a complex pharmaceutical market like Georgia’s will most likely fail to meet its objective i.e. a reduction in costs to the public, and improved access to pharmaceuticals. Therefore, the government needs to immediately implement a multi-pronged policy to better address the issue.
This policy should include the following:
- The introduction of reference pricing on the market – the government can achieve this by learning from other countries’ (high/low/middle-income) experiences and best practices. Through observing others’ experiences, the most appropriate reference pricing methodology can be utilized to further facilitate the regulation of drug prices in the country.
- Encourage the use of generic prescription drugs and enforce the generic substitution in the prescription of medicines.
- Introduce strict rules and controls for drug promotion, marketing, education, and sponsorship gifts to doctors.
- Enhance pharmaceutical market monitoring to adequately adjust for weaknesses in the policy or its implementation.
The full report is available here.
About the Study
The study was conducted using World Health Organization (WHO) standard methodology. The survey looked at the prices and mark-ups of 52 medicines (brand-name medicines and their generic equivalents) in six regions of Georgia.
CIF has been conducting the PPA study since 2009. The results of the study’s previous waves were released in 2010, 2011, 2012 and 2014.
Latest News
Integrated Bio-behavioral surveillance and population size estimation survey among Female Sex Workers in Tbilisi and Batumi, Georgia, in 2024
We’re excited to announce the completion of a significant study conducted in 2024 that estimates HIV-related risk behaviors and HIV and syphilis prevalence among female sex workers (FSWs) in Tbilisi and Batumi, Georgia. Building on surveys initiated in 2002, the…
Curatio International Foundation at Eighth Global Symposium on Health Systems Research (HSR2024)
During 18-22 November 2024 in Nagasaki, Japan will be hosting the Eighth Global Symposium on Health Systems Research. Curatio International Foundation will actively participate in the symposium, presenting research outcomes and acting as panelists on the different sessions. Read more…
“The Informatics and Data Science for Public Health: Sustainment Plan for Skilled Labor Force Development”
Curatio International Foundation publishes the report “The Informatics and Data Science for Public Health: Sustainment Plan for Skilled Labor Force Development”. The report highlights the assessment results conducted in Georgia, Kazakhstan and Moldova, with the aim to evaluate market, supply…
Janina Stauke from the London School of Hygiene and Tropical Medicine shares her internship experience
Janina Stauke from LSHTM was a CIF Intern during the 2024 Summer internship program, where she contributed to a project focused on understanding why individuals experience impoverishment due to health spending. In her role, conducted comprehensive literature review and has…
Jolly Mae Catalan fromUniversité Libre de Bruxelles shares her internship experience
Jolly Mae Catalan was a CIF Intern during the 2024 Summer internship program, where she contributed to a project focused on understanding why individuals experience impoverishment due to health spending. In her role, Jolly examined the impact of the accessibility and…
Georgia’s Journey to Integrating Rehabilitation Services into the Health System: Insights and Lessons
This Learning Brief captures Georgia’s journey in integrating rehabilitation services into its health system, offering a detailed account of the strategies, actions, and lessons learned from this transformation. Supported by USAID, Accelerator and Curatio International Foundation, the project aimed to…
Report on Rehabilitation Data Flow in Georgia’s Health Information System
The Curatio International Foundation (CIF) publishes report on Rehabilitation Data Flow in Georgia’s Health Information System (HIS). This study investigates how rehabilitation information currently flows within Georgia’s HIS, identifies strengths and shortcomings, and provides recommendations for enhancing the rehabilitation information…
Georgia: a primary health care case study in the context of the COVID-19 pandemic
The case study examines primary health care (PHC) in the context of the COVID-19 pandemic in Georgia between January 2020 and July 2021. The Astana PHC components are used to consider integrated integrated primary care and essential public health functions,…
Adult vaccination in Asia and the Pacific
Curatio International Foundation in partnership with Asian Development Bank publishes a report entitled “Adult vaccination in Asia and the Pacific- policies, financial needs, and fiscal impacts” Immunization is one of the most cost-effective tools for improving health and well-being. The coronavirus…
Assessment of the Quality of Maternal and Neonatal Services in Montenegro
Curatio International Foundation (CIF) publishes a report on the Assessment of the Quality of Maternal and Newborn Care in Montenegro, a project supported by UNICEF and in collaboration with the Montenegro’s Ministry of Health. This initiative enriched by CIF’s expertise,…
Call for Internship 2024
About host organization Curatio International Foundation (CIF) is a not-for-profit, non-governmental organization with a mission to improve health through health policy and systems strengthening. CIF’s work is underpinned by three core values: hearing needs; building on local strengths; and delivering…
Georgian state rehabilitation program: implementation research study report
The Curatio International Foundation (CIF) publishes implementation research study report, which aims to document the initial results and challenges of Georgia’s state rehabilitation program, to inform and facilitate the program’s timely scale-up beyond the initial package of interventions. This study was conducted by…
NEW Barometer, study focusing on the pharmaceutical sector
The Curatio International Foundation publishes a new Barometer, a study is focusing on the pharmaceutical sector. The purpose of the study is to examine the inflation on healthcare with a focus on pharmaceuticals. The study includes the data of the…
Strengthening Health Systems for Accessible Rehabilitation Services in Georgia
The Curatio International Foundation, in collaboration with R4D (Results for Development) and USAID’s ‘Health Systems Strengthening Accelerator,’ conducted a stakeholder meeting on September 15th as part of the ‘Strengthening Health Systems for Accessible Rehabilitation Services in Georgia’ project. During the…
Linked’s workshop on HPV vaccine introduction and scale up, held on July 11-12th, 2023
In a significant step towards combating the impact of Human Papillomavirus (HPV) infections, in partnership with R4D, CIF organized a two-day in-person workshop in Istanbul, Turkey, to address the challenges faced by Middle-Income Countries (MICs) in HPV vaccine introduction and…
Training program focusing on interdisciplinary evaluation of rehabilitation interventions and patient outcomes
The Curatio International Foundation conducted a training program focusing on interdisciplinary evaluation of rehabilitation interventions and patient outcomes. This initiative was carried out as a part of the “Strengthening Health Systems for Accessible Rehabilitation Services in Georgia” project, in collaboration with…
Unlocking Success Through Learning: Workshop on Strengthening HR Capacity and Performance Management in Immunization
Multiple factors affect health worker performance and, by extension, immunization program performance. But what are the most important factors that lead to health workers performing well? During 6-7 June, about 30 participants, including government representatives and country partners from Armenia,…
Promote evidence-based policies in the pharmaceutical sector by generating evidence and fostering civic engagement
Curatio International Foundation (CIF) is running the project entitled “Promote evidence-based policies in the pharmaceutical sector by generating evidence and fostering civic engagement” in partnership with Social Equation Hub (SEH). In the frame of the program on June 22 –…
CIF and the Results for Development / Accelerator combined their expertise to co-author an insightful blog, shedding light on Georgia’s commendable efforts to overcome limited data challenges and develop evidence-based policies for financing rehabilitation services
The Ministry of Internally Displaced Persons from the Occupied Territories, Labor, Health, and Social Affairs in Georgia has taken a significant step in extending financial protection for rehabilitation services through the Universal Health Coverage Program. With support from the Health…
Culminating event – Building Institutional Capacity for Health Policy and Systems Research and Delivery science (BIRD) in six WHO Regions
3-day culminating event was conducted in Beirut, Lebanon in the frame of “Building Institutional Capacity for Health Policy and Systems Research and Delivery science (BIRD) in six WHO Regions” program. The meeting aimed to reflect on achievements and learnings and…
Report on Phased (Stepwise) Plan for the Capability Development of the Priority Rehabilitation Services
The Inclusive Development Hub of USAID’s Bureau for Development, Democracy, and Innovation has partnered with the Accelerator to support countries in strengthening and integrating rehabilitation services in the health systems. Georgia was selected as one of the priority countries for…
Report on prioritization of rehabilitation services in Georgia
The Inclusive Development Hub of USAID’s Bureau for Development, Democracy, and Innovation has partnered with the Accelerator to support countries in strengthening and integrating rehabilitation services in the health systems. Georgia was selected as one of the priority countries for…
Report on Rehabilitation Service Costing and Budgeting
The Inclusive Development Hub of USAID’s Bureau for Development, Democracy, and Innovation has partnered with the Accelerator to support countries in strengthening and integrating rehabilitation services in the health systems. Georgia was selected as one of the priority countries for…
Mental health of young people during the COVID-19 pandemic
The COVID-19 pandemic has taken its toll on the mental health and wellbeing of the population globally. Even two years after the COVID-19 Pandemic breakout, the impact on the mental health of populations globally, particularly children and young people, is…
Promote evidence-based policies in the pharmaceutical sector by generating evidence
Duration From: September 2022 To March 2024 Introduction and Overview The research project aims to promote evidence-based policies in the pharmaceutical sector by generating evidence and fostering civil engagement. The project will achieve the aim through two objectives: Investigate the…
Mandatory Vaccination and Green Passes – Review of International Experience
Globally, the COVID-19 vaccination started at the end of 2020 and continues at a different paces in various countries. As of December 7 2021, 8 billion doses of vaccine have been administered worldwide, and 45% of the world’s population has…
Sustaining Public Health Gains after Donor Transition: What can we learn about Georgia?
The CIF research team publishes Synthesis Report entitled: “Sustaining Public Health Gains after Donor Transition: What can we learn about Georgia?”. The research project aimed to comprehensively evaluate donor transitions that took place in Georgia in the immunization program (NIP) after introducing…
Curatio International Foundation at Seventh Global Symposium on Health Systems Research (HSR2022)
During October 31 – November 04, 2022, Bogota, Colombia hosted the Seventh Global Symposium on Health Systems Research. Research team of Curatio International Foundation actively participated in the symposium, organized couple satellite sessions and presented research outcomes to…
Curatio International Foundation at Global Symposium on Health Systems Research
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
EECA HIV Sustainability Summit 2022 in Tbilisi
On September 26 – 28, EECA HIV Sustainability Summit 2022 took place in Tbilisi, Georgia, which aimed to mobilize national, regional, and international stakeholders and create a platform for the high-level dialogue on strategic vision and priority actions required to…
Study report: Adaptations made in TB response during Covid-19 pandemic in Georgia
The CIF research team publishes study report entitled “What adaptations were made in TB response during Covid-19 pandemic in Georgia: health systems perspective on the implications for TB case detection and treatment provision”. The research project aimed to study how the state…
New review of our recent study on Immunization in Kazakhstan
„The report offers the most clear, comprehensive layout of the problems of planning and providing immunization in the Republic of Kazakhstan and offers detailed solutions. In essence, the document is a step-by-step guide to fixing the situation and must be read…
New case study: Sustaining effective coverage with Opioid Substitution Therapy (OST) in Georgia in the context of transition from external assistance
The CIF research team publishes new case study entitled “Sustaining effective coverage with Opioid Substitution Therapy (OST) in Georgia in the context of transition from external assistance”. The study aims to better understand how and why Georgia was able to…
New case study: National Immunisation Program Transition from external assistance
The CIF research team publishes a new case study entitled “National Immunization Program (NIP) Transition from external assistance – Case Study from Georgia”. The purpose of this case study is to understand the transition of the National Immunization Program (NIP)…
“Strengthening Health Systems for Accessible Rehabilitation Services in Georgia” – Workshop
On August 01, 2022, Curatio International Foundation in the frame of the project: “Strengthening Health Systems for Accessible Rehabilitation Services in Georgia” held a workshop with relevant stakeholders. The purpose of the workshop was to present to the audience and…
Strengthening the Delivery of Immunisation Services Through PHC Platforms-Workshop
On July 26-27, a Linked workshop on Strengthening the Delivery of Immunisation Services Through Primary Healthcare (PHC) Platforms was successfully held by Curatio International Foundation. The collaborative learning session was held with the involvement of seven countries across the Europe-Central…
Call for internship 2022
About host organization Curatio International Foundation (CIF) is a not-for-profit, non-governmental organization with a mission to improve health through better functioning health systems. CIF’s work is underpinned by three core values: Hearing Needs, Building on Local Strength; and Delivering Innovative…
New Article published in the journal Frontiers in Public Health
In June 2022, a multidisciplinary open-access journal Frontiers in Public Health published an article: The State of Public health education and science during and after the fall of the Soviet Union: achievements, remaining challenges, and future priorities, by George Gotsadze,…
New Paper: A transdiagnostic psychosocial prevention-intervention service for young people in the Republic of Georgia
CIF researchers in collaboration with partners from GIP-Tbilisi and Cardiff University published new scientific article in the European Journal of Psychotraumatology. The article entitled “A transdiagnostic psychosocial prevention-intervention service for young people in the Republic of Georgia: early results of…
Vaccine Procurement and Supply for the Expanded Program of Immunization in Kazakhstan
In May 2022 UNITED NATIONS CHILDREN’S FUND (UNICEF) Kazakhstan published a report entitled: “Vaccine Procurement and Supply for the Expanded Program of Immunization in Kazakhstan: Gaps and Challenges for Action”, authored by Dr. George Gotsadze and Dr. David Sulaberidze, both…
Prevention of Addiction and Mental Health in Adolescents in Georgia (PAMAd) – Workshop
On May 19 – 20, 2022, Curatio International Foundation held a workshop in the frame of the research project:” Prevention of Addiction and Mental Health in Adolescents in Georgia (PAMAd)”. The project aims to improve the health and well-being of adolescents…
Workshop to discuss the risk assessment of future TB migrants
Curatio International Foundation held a workshop aiming to discuss: “The risk assessment of future TB migrants and offer a mechanism to reduce those risks based on advanced international experience” considering the Georgian context. Various stakeholders attended the meeting, from both…
Webinar : Matters of Scale and Integration in Digital Health Ecosystems
On February 28th, Curatio International Foundation participated in the webinar “Matters of Scale and Integration in Digital Health Ecosystems”, a part of the Digital Dialogue Series on Mixed Health Systems held by. During the webinar, the president of Curatio International…
Immunisation Action Network
Curatio International Foundation (CIF) in partnership with The Institute for Health Policy (IHP), and Results for Development (R4D) will co-lead the Linked Immunisation Action Network. CIF will serve as the focal point for six countries in the Europe and Central Asia region,…
Data Analysis and Synthesis Workshop – analyzing the implications of the structure of Georgia’s private healthcare market for quality and accessibility
On January 17-21, 2022, the Curatio International Foundation held Data Analysis and Synthesis Workshop in the frame of the project: “Concentration and fragmentation: analyzing the implications of the structure of Georgia’s private healthcare market for quality and accessibility (ConFrag)“. The…
External Reference Pricing Policy: A Possible Pharmaceutical Price Regulation Policy in Georgia
rmaceutical expenditure is a heavy financial burden and one of the factors of the impoverishment of the Georgian population. One of the leading factors contributing to the particularly high medicine expenditure in Georgia is a significant gap in pharmaceutical policy…
Rapid reviews of health policy and systems evidence
Rapid reviews of health policy and systems evidence provide relevant and actionable evidence at every step of the decision-making process. The Alliance established the Embedding Rapid Reviews in Health Systems Decision-Making (ERA) initiative to build rapid review production directly within…
Paper: Soviet legacy is still pervasive in health policy and systems research in the post-Soviet states
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Strategic Fellowship – series of training courses for students
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Georgian NIP faces challenges in sustaining the outcomes achieved
On October 18, 2021, The Learning Network for Countries in Transition (LNCT) published new case study lessons learned: Lessons Learned from Georgia’s Experience Transitioning From GAVI support authored by: Ivdity Chikovani, Curatio International Foundation, Georgia Paata Imnadze, Deputy General Director,…
Enroll to the CIF’s Strategic Fellowship Programme
Curatio International Foundation in collaboration with the American University of Beirut, announces an educational Fellowship Program for professionals interested and active in the field of healthcare, for people who want to become advocates for change. The program is being implemented…
Article: How do participatory methods shape policy? Applying a realist approach to the formulation of a new tuberculosis policy in Georgia
The paper presents the iterative process of participatory multistakeholder engagement that informed the development of a new national tuberculosis (TB) policy in Georgia, and the lessons learned. The article was published in BMJ Journals, authored by researchers from Curatio International…
Bundled Payment Methods: An Alternative Payment Method to Contain Healthcare Costs in Georgia
Containing healthcare costs remains one of the priority topics of the Georgia health system from the perspectives of both the public and private sector. Government expenditure on health has been increasing between 2013-2019 after the introduction of the Universal Health…
HOW TO MAINTAIN ROUTINE IMMUNIZATION DURING COVID-19? EXPERIENCES FROM ARMENIA, GEORGIA, AND UZBEKISTAN
Author: Eka Paatashvili Over this last year, countries around the world have been forced to focus most of their efforts on fighting the COVID-19 pandemic, potentially leaving other health priorities, including routine immunization, to fall by the wayside. To understand…
DRUG CHECKING: An Essential Response to Emerging Harm Reduction Needs
Georgia has observed increasing trends of use of new psychoactive substances (NPS), as well as increased rates of non-injecting drug use in recreational settings, based on the latest available evidence. Additionally, the circulation of unknown NPS poses significant risks to…
GEORGIA COVID-19 VACCINE COMMUNICATIONS CAMPAIGN TO ADDRESS HESITANCY ISSUES
Authors: Ina Charkviani, Journalist and Communications Specialist, Curatio International Foundation and Lela Sturua, Head of Non-Communicable Disease Department, National Center for Disease Control (NCDC) of Georgia As the COVID-19 pandemic continues to shake the globe, signs of hope are beginning…
Supporting Evidence-Informed Policy making and Action in Six WHO Regions
On March 31, 2021, the Curatio International Foundation participated in HSR2020 final phase session on Innovative Models of Institutional Arrangements to Support Evidence-Informed Policy making and Action in Six WHO Regions. In this session, Curatio International Foundation as a European…
Georgian Healthcare Barometer XIV Wave The analysis of financial stability and risks in healthcare
Curatio International Foundation publishes the new 14th wave of the healthcare barometer which analyzes the financial situation and risks in Georgian healthcare sector. The new barometer has been prepared under the framework of cooperation between the Curatio International Foundation and…
Call for educational Strategic Policy Fellowship Program
Curatio International Foundation, in collaboration with the American University of Beirut, is announcing an educational Strategic Policy Fellowship Program for professionals working and active in the health field, for people who want to become advocates for change. The program is…
We are pleased to announce that The Sixth Global Symposium on Health Systems Research (HSR2020) has opened
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Discussing interim results of research project: Prevention of Addiction and Mental Health in Adolescents in Georgia (PAMAd)
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Effects of Pay for Performance on utilization and quality of care among Primary Health Care providers in Middle and High-Income countries
Curatio International Foundation experts reviewed Pay for Performance (P4P) effectiveness on utilization and quality of primary health care in private settings in middle-income and high- income countries. About P4P Pay for Performance (P4P) is a relatively new strategy aiming at…
Does pay for performance work to improve immunization coverage?
Authors: Ivdity Chikovani Pay for Performance (P4P) is a financing mechanism that has flourished in recent years as countries look for innovative ways to improve coverage of health services. P4P can be employed as an approach to incentivize health care…
CAN SOCIAL MEDIA MONITORING LEAD TO IMPROVED PERCEPTIONS ABOUT IMMUNIZATION?
Authors: Eka Paatashvili, Gayane Sahakyan (MoH, Armenia), Svetlana Grigoryan (MoH, Armenia) The recent COVID-19 crisis has shown how social media can be used successfully for community engagement and emotional support, as well as for providing the latest global evidence. Yet,…
The first phase of the joint fellowship program of the Curatio International Foundation and the Knowledge to Policy Center (K2P) at the American University of Beirut has been successfully implemented
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
LNCT WEBINAR: Incremental Costs of Routine Immunization, Campaigns, and Outreach Services During COVID-19
CLICK HERE TO REGISTER FOR THE WEBINAR Please join us on Thursday, July 16, 2020, for LNCT webinar: Incremental Costs of Routine Immunization, Campaigns, and Outreach Services During COVID-19. The COVID-19 pandemic has disrupted immunization services critical to the prevention of vaccine preventable…
LNCT Webinar: Assessing Bottlenecks to Adequate and Predictable Vaccine Financing
Please join us on Tuesday, June 23, 2020 for LNCT webinar, Assessing Bottlenecks to Adequate and Predictable Vaccine Financing. Diagnosing the root cause of constraints affecting the adequacy and predictability of vaccine financing is an important part of the Gavi…
LNCT WEBINAR: Designing Behavioural Strategies for Immunization in a Covid-19 Context
Please join us on Thursday, May 21, 2020 for an LNCT webinar, ‘Designing Behavioral Strategies for Immunization in a Covid-19 Context.’ Hosted by Common Thread, this webinar will review the Covid-19 context that many GAVI countries now find themselves in….
Concentration and fragmentation: analyzing the implications of the structure of Georgia’s private healthcare market for quality and accessibility (ConFrag)
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
LNCT Discussion Group: COVID-19 Impact on Immunization Programs
With the COVID-19 pandemic straining health systems around the world, LNCT would like to support countries to minimize potential negative effects on their immunization programs. To understand the challenges posed by COVID-19 on immunization in LNCT countries and exchange on…
#COVID19 – Evidence and Policymaking: Personal Reflections from an LMIC Setting
by George Gotsadze, President of Curatio International Foundation and Executive Director of Health Systems Global As I embark on writing this blog, the global outbreak of COVID-19 is posing a growing threat to the health and well-being of our societies. The unfolding…
The COVID-19 epidemic in Georgia Projections and Policy Options
Curatio International Foundation continues to support the Georgia government by providing evidence for evidence-informed decision making. Current effort – a Rapid Response document – represents an evidence synthesis about effective measures to respond to the COVID-19 epidemic. Since the epidemic…
Modeling of Four Possible Scenarios of COVID-19 epidemics in Georgia
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Application for Summer Internship program is closed
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
LNCT Webinar: Key Considerations for Integrating Immunization with Other Primary Health Care Services
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Georgia Sharing knowledge to Armenia to strengthen immunization legislation
Armenia and Georgia, members of Learning Network for Countries in Transition (LNCT) participated in the twinning program on immunization legislation In February 2020. The twinning program was hosted by Curatio International Foundation. Armenian delegation represented by the Ministry of Health…
Call for Interns as researchers is now open
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Improving access to pharmaceuticals in Georgia
Curatio International Foundation conducted a policy dialogue meeting about Pharmaceutical Pricing Policies to improve the population’s access to pharmaceuticals in Georgia. The meeting was attended by various stakeholders from government agencies, non-governmental organizations, pharmaceutical companies associations. Field experts were also…
LNCT Webinar: Addressing Vaccine Hesitancy Challenges
Please join us on February 6th for a webinar featuring country experiences related to vaccine hesitancy challenges. Panelists from the London School of Hygiene and Tropical Medicine will present a summary of the key issues and lessons learned during LNCT’s vaccine…
Project on “Technical Assistance Using Modern Technology for TB Prevention, Diagnosis, and Increased Quality Treatment” was closed
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Dialogue on Pharmaceutical pricing policies to improve the population’s access to pharmaceuticals in Georgia
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
LNCT Webinar: Implementing a High Performing Immunization Program within the Context of National Health Insurance: What can we Learn from Thailand?
Please join us on Wednesday, December 11th for LNCT webinar, ‘Implementing a High Performing Immunization Program within the Context of National Health Insurance: What can we Learn from Thailand?’ Since the adoption of the National Health Security Act in 2002, all Thai citizens…
LNCT Webinar: Strengthening Public-Private Engagement for Immunization Delivery
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Implementing new research: Prevention of Addiction and Mental Health in Adolescents in Georgia
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Georgia’s introduction of the Hexavalent vaccine: Lessons on successful procurement and advocacy
Authors: Eka Paatashvili and Vladimer Getia As countries transition from Gavi support, sustaining access to affordable and high-quality vaccines is one of many challenges they face. Fully self-financing countries need to maintain their immunization programs while also committing increased funds…
Georgia Primary Health Care Profile: 6 Year after UHC program introduction
Curatio International Foundation publishes Georgia Primary Health Care (PHC) profile. Georgia PHC profile was prepared based on Primary Health Care Vital Signs framework developed by the Primary Health Care Performance Initiative (PHCPI). PHCPI is a partnership dedicated to transform the…
National consultations to propose a new model of TB funding
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
HSR2020: RE-IMAGINING HEALTH SYSTEMS FOR BETTER HEALTH AND SOCIAL JUSTICE
We are pleased to announce the theme and sub-themes for the Sixth Global Symposium on Health Systems Research in Dubai in November 2020: “Re-imagining health systems for better health and social justice” Ten years on from the First Global Symposium on Health Systems…
CALL FOR MENTEES: PUBLICATION MENTORSHIP FOR FIRST-TIME WOMEN AUTHORS IN THE FIELD OF HPSR
Health Systems Global (HSG) and The Alliance for Health Policy and Systems Research (AHPSR) are committed to supporting individual capacity for the conduct and uptake of health policy and systems research (HPSR). Peer reviewed publications are important for communicating scientific…
A pilot of a new intervention launched to Improve adherence to TB treatment and its outcomes in Georgia
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Training for epidemiologists and health workers on TB contact tracing new guideline
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Workshop on using modern technology for TB prevention, diagnosis and increased quality treatment
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
K2P Mentorship Program on Building Institutional Capacity on Evidence Informed Policy Making
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Training on using Research Evidence for Policy Making
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Doing embedded development and research – reflections on the start of the Results4TB programme
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Healthcare Advocacy Coalition (HAC) for Human Oriented Healthcare
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Introductory Meeting on the project ‘Embedding Rapid Reviews in Health Policy-Making’
On November 5, the Committee on Health and Social Affairs of the Parliament of Georgia will host an introductory meeting on the project Embedding Rapid Reviews in Health Policy-Making. The project is jointly implemented by the “Curatio International Foundation” and…
Taavy Miller from University of North Carolina shares her internship experience
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Building Institutional Capacity for HPSR and Delivery Science- CIF is Europe region HUB
General Overview The evidence-informed decision making in health still remains a major challenge. To strengthen institutional capacity in different countries around the globe the Alliance for Health Policy and Systems Research (AHPSR) launched the new program to strengthen the capacity…
Inter-regional workshop in preparation for transitioning towards domestic financing in TB, HIV and Malaria programmes
During October 17-19 the WHO Regional Office for Europe in collaboration with WHO headquarters, GFATM and USAID organised a workshop in Tbilisi to provide countries a platform for dialogue on sustainability and transition issues, and to further define regional and…
Memorandum of Cooperation between the Health and Social Issues Committee of the Parliament of Georgia and Curatio International Foundation
Chairman of the Health Care and Social Issues Committee of the Parliament of Georgia Akaki Zoidze and Curatio International Foundation President George Gotsadze signed a memorandum of cooperation. The collaboration aims to support evidence usage by the parliament when dealing with the…
Embedding Rapid Reviews in Health Systems Decision-Making (ERA)
General Overview As a result of the constitutional amendments passed at the end of 2017, Georgia became a parliamentary republic. It increased the role of the legislature in policy development and supervision. As a result of these amendments, the Committee…
Curatio International Foundation at the Global Symposium on Health Systems Research
During October 8-12, 2018 Liverpool hosted the Fifth Global Symposium on Health Systems Research. Curatio International Foundation, as a secretariat of Health Systems Global, organzied the event for the second time in close collaboration with HSG’s partners. Over 2300 participants from…
The civil society gathered for the fourth time to discuss healthcare system challenges in Georgia
On September 27-29, the fourth work meeting of a civil society was held in Kachreti. Representatives of a civil society, media, and academia convene under the framework of the joint project of Curatio International Foundation and Open Society Foundation. The…
Project: HIV risk behavior among Men who have Sex with Men – Bio-Behavioral Surveillance Survey and Population Size Estimation
Introduction and Overview Georgia is among the countries with low HIV/AIDS prevalence but with a high potential for the development of a widespread epidemic. From the early years of the epidemic injecting drug use was the major route for HIV transmission,…
Curatio International Foundation at AIDS2018
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Big Pharma Greed and Artificial Prices – Knocking on Door to Limit Access to HIV Medicines in Georgia
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Civil society is gathering for the third time to hold a discussion about the healthcare
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Webinar: Mapping and consensus of global competencies set for the field of HPSR: A progress update and HSG round table discussion
This webinar took place on July 11th, 2018. If you missed it or would like to listen to it again, you can watch the recording. Are you involved with designing HPSR training programs? Do you create course content for HPSR training?…
Webinar: Vaccine Forecasting and Budgeting Tools and Best Practices
This webinar took place on July 2, 2018. However, if you missed the webinar or would like to listen to it again, you can watch the recording and download the slides. Participants will learn about existing vaccine forecasting and budgeting tools and best…
Technical Assistance for evaluation of transition readiness and preparation of Transition and Sustainability Plan for Global Fund-supported programs in Tajikistan
Introduction and Overview In June 2018 CIF initiated a new project with the financial support of The Global Fund. The overall goal of the CIF assignment is to support the Country Coordination Mechanism of Tajikistan (CCM) in assessing country preparedness…
Technical Assistance for the preparation of Transition and Sustainability Plan for HIV program in Philippines
Introduction and Overview Since May 2018 Curatio International Foundation implements a technical assistance for the Philippines to prepare Transition and Sustainability Plan for HIV program. The overall goal of the given assignment is to support the Department of Health and…
Discussing the accessibility of medicines in Georgia
Curatio International Foundation, within the framework of the ongoing project: “Engaging civil society in decision-making and monitoring in Georgian healthcare sector” supports strengthening and consolidating civil society representatives interested in health care for advocacy on health issues. To this end,…
Webinar: Integrating gender into health system strengthening in conflict and crisis-affected settings; what’s in our toolkit?
This webinar took place on June 22, 2018. However, if you missed the webinar or would like to listen to it again, you can watch the recording and download the slides. In 2016, HSG held the first webinar on gender, asking the question, “What…
Article: Barriers to mental health care utilization among internally displaced persons in the republic of Georgia: a rapid appraisal study
The new paper identifies the health system barriers leading to low rates of utilization of mental health services among internally displaced people (IDP) with mental disorders. The paper was published in BMC Health Services Research authored by Adrianna Murphy, Ivdity…
Why Georgians second-guess their doctors – Deregulation has left Georgian medical care something many Georgians would rather avoid
Full Article is available on Eurasianet ” […] Last year, Giorgi Tsiklauri, 24, was diagnosed with leukemia at a hospital in Tbilisi and was about to undergo chemotherapy, but he decided to seek a second opinion in Turkey. As it…
Ara Srinagesh from New York University Shares her Internship Experience
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Webinar on The peer review process – what happens when you send your manuscript to a journal
This webinar took place on April 23, 2018. However, if you missed the session or want to listen to it again, you can watch the recording. Have you ever wondered what the journal editor’s viewpoint is on your article, or what…
Webinar on Improving Quality of Care during Childbirth: Learnings and Next Steps from the BetterBirth Trial
This webinar took place on April 24, 2018. However, if you missed the session or want to listen to it again, you can watch the recording. Join the webinar organized by HSG Thematic Working Group Quality in Universal Health and Healthcare. During…
Civil Society is Being United to Discuss Healthcare System Issues
Curatio International Foundation, within the framework of the ongoing project: “Engaging civil society in decision-making and monitoring in Georgian healthcare sector” along with the Open Society Network supports strengthening and consolidating civil society representatives interested in health care for advocacy…
Curatio International Foundation for a TB-Free World
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Summer Internship Program is now open
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Closing Project: Tuberculosis Community Systems Strengthening in Georgia
The Curatio International Foundation has fulfilled a Tuberculosis Community Systems Strengthening (TBCSS) Project in Georgia, funded by the Stop TB partnership in the frame of Challenge Facility for Civil Society (CFCS) round 7 program. The goal for the project was…
Primary Health Care Systems: Georgia case study
Curatio International Foundation publishes Georgia case study of primary health care system (PRIMASYS). The PRIMASYS case study covers key aspects of primary health care system, including policy development and implementation, financing, integration of primary health care into comprehensive health systems,…
Integrated Bio-behavioral surveillance and population size estimation survey among Female Sex Workers in Tbilisi and Batumi, Georgia
This study represents the subsequent wave of Bio-Behavioral Surveillance Surveys (BBS) surveys undertaken among Female Sex Workers (FSW) since 2002. The current study was conducted in 2017 using the Time-Location Sampling technique and 350 FSWs was recruited in total in…
Applying a Health Policy and Systems Research lens to Human Resources for Health: Capacity building, leadership and politics
This webinar took place on March 14, 2018. However, if you missed the webinar or want to listen to it again, you can watch the recording and download the slides. How do we study ‘invisible’ concepts such as politics and power as they…
CIF hosts Aradhana Srinagesh throughout the winter internship program
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Empowering civil society for engagement in and monitoring the decision making in health sector in Georgia
Introduction and Overview The project aims to strengthen CSOs working on Health Systems to participate in the decision-making process, to assume watchdog functions, monitor enforcement of policies and advocate for better health for all. The project is funded by Open Society…
Curatio International Foundation presented BBS and PSE study findings at the Civil Society Forum
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Article: Barriers to delivering mental health services in Georgia with an economic and financial focus: informing policy and acting on evidence
A new paper discusses the economic and financial barriers to delivering mental health services in Georgia and assessing the opportunities for reform that can support the development of strategies for change. The article was published in BMC Health Services Research,…
The Interview on population size and Human Immunodeficiency Virus risk behaviors of People who Inject Drugs in Georgia
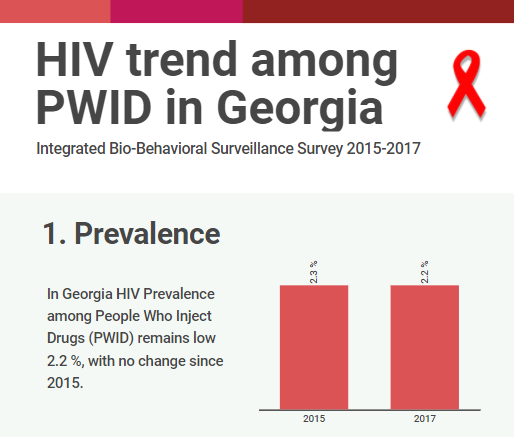
The interview is based on the latest wave of the integrated Bio- behavioral surveillance survey conducted People Who Inject drugs (PWID) in 7 cities of Georgia. The research aims to measure the prevalence of Human Immunodeficiency Virus (HIV) and Hepatitis…
Population Size Estimation of People who Inject Drugs in Georgia 2016-2017
Bemoni Public Union together with Curatio International Foundation conducted a population size estimation study among injecting drug users in Georgia during 2016-2017. Also available: HIV risk and prevention behaviors among People Who Inject Drugs in six cities, Georgia, 2017 This…
HIV risk and prevention behaviors among People Who Inject Drugs in seven cities of Georgia, 2017
Curatio International Foundation together with Bemoni Public Union has conducted HIV prevalence and risk behaviors survey among People Who Inject Drugs in Georgia. Also available: Population Size Estimation of People who Inject Drugs in Georgia 2016-2017 Current study represents the…
Conference paper: The Study of Barriers and Facilitators to Adherence to Treatment among Drug Resistant Tuberculosis Patients in Georgia to Inform Policy Decision
The abstract has been submitted and accept for oral presentation at The Union 2017 – 48th Union World Conference on Lung Health, 11 – 14 October, 2017 Guadalajara, Mexico The study outlines different health system factors as long as some…
Article: Human immunodeficiency virus prevalence and risk determinants among people who inject drugs in the Republic of Georgia
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Conference paper about realist evaluation: Informing policy, assessing its effects and understanding how it works for improved Tuberculosis management in Georgia
In the last week of October, 2017 Brisbane, Australia hosted International Conference for Realist Research Evaluation and Synthesis – Realist2017. Realist evaluation is complex sensitive approach and is useful for decision makers because rather responding the question “does the intervention…
Georgian Healthcare and its Challenges: Healthcare Expert George Gotsadze will host the lecture
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
17 years in Curatio International Foundation: President Ketevan Chkhatarashvili to Leave Organization
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Be ready for the Best Internship Experience
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Apply for Our Winter Internship Program
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Georgian Solution for a Post-Soviet TB Program: Can Integration into Primary Health Care Improve TB Care?
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Moving Forward: Second PT workshop with TB stakeholders in Georgia
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Article: Determinants analysis of outpatient service utilization in Georgia: can the approach help inform benefit package design?
Curatio International Foundation conducted secondary data analyses of Health Service Utilization and Expenditure survey (2 waves), conducted by Ministry of Labor Health and Social Affairs of Georgia, supported by WHO and The World Bank. We studied factors that impact utilization…
Designing and evaluating provider results-based financing for tuberculosis care in Georgia (RBF4TB)
Introduction and Overview CIF in partnership with Queen Margaret University (UK), London School of Hygiene and Tropical Medicine (UK) and Antwerp Institute of Tropical Medicine (Belgium) is implementing a study “Designing and evaluating provider results-based financing for tuberculosis care in…
Forbes Georgia: The Importance of Evidence in Decision Making
In February 2017, CIF director George Gotsadze talked with Forbes Georgia. The interview discusses the role of effective management in health systems and impact of innovations in the field. In the article George speaks about CIF experience serving as HSG Secretariat. Read more…
Barriers and Facilitators to Adherence to Treatment Among Drug Resistant TB Patients in Georgia
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Transition Preparedness Assessment
Sustainability of national HIV and TB programs gains importance in light of recent changes in the global health landscape when external funders are redirecting resources to poorer states while phasing out from middle-income countries. Objectively evaluation of the country transition…
New Study Findings About Tuberculosis
Curatio International Foundation together with the Partnership for Research and Action for Health organized a meeting at the National Center for Disease Control and Public Health on 26th of December, where two different study findings were represented. Studies aimed to…
Meet CIF in Vancouver at HSR2016
Curatio International Foundation will be featured at Health Systems Research 4th Symposium to be held in Vancouver, Canada November 14 -18, 2016. CIF experts will discuss several important health system-related issues and share particular knowledge with a global audience. We…
Curatio International Foundation: Transition and Sustainability Portfolio
As middle-income countries experience economic growth and increased government spending potential on healthcare, donors have begun decreasing their support under the assumption those countries have sufficient physical space to support and sustain their health programs. Countries in Eastern Europe and…
Article: Privilege and inclusivity in shaping Global Health agendas
Health Policy and Planning published an article Privilege and inclusivity in shaping Global Health agendas. CIF director George Gotsadze co-authors the paper together with Kabir Sheikh, Sara Bennett and Fadi el Jardali. The article discusses lack of inclusivity in Global Health…
Eastern Europe and Central Asia Regional Sustainability and Transition Coordination Summit 20-21 October, 2016 Vilnius, Lithuania
In recent years there has been a significant decrease in funding from international and bilateral donors, including The Global Fund (TGF) to support HIV and TB response in middle income countries across Eastern Europe and Central Asia (EECA). This raises…
Internship Announcement, Winter 2016-17
The Curatio International Foundation has released the results of the fifth wave of the Pharmaceutical Price and Availability (PPA) study in Georgia. The study set out to generate further evidence regarding pharmaceutical prices and availability in the country through the...
Evaluation of UNICEF’s Contribution in Central and Eastern European Five Countries
Curatio International Foundation conducted an evaluation of UNICEF’s contribution to the reduction of under 5 mortality in five countries: Kazakhstan, Kyrgyzstan, Moldova, Serbia, and Uzbekistan. The evaluation covered 12 years from 2000 – 2012 and was performed in 2014-2015. UNICEF’s…
CIF researchers represented on Global Conference AIDS2016
CIF researchers Ivdity Chikovani and Lela Sulaberidze participated in AIDS 2016 conference that was held in Durban, South Africa in July 2016. The conference assembled over 18,000 delegates from around the world. Scientists, policymakers, world leaders, and people living with…
Assessment of GAVI Alliance HSS support to Tajikistan
In August 2014, Curatio International Foundation conducted an assessment of GAVI Alliance HSS support to Tajikstan to provide solid evidence of to what extent the support achieved its objectives and contributed to strengthen the health system of the country. The assessment…
Final Evaluation of Gavi’s Support to Albania
After the conclusion of Gavi’s support period (2014) to the Albania, Curatio International Foundation conducted the evaluation study and assessed financial and programmatic sustainability through an in-depth analysis of Albania’s experiences and immunization programme performance before, during and after the…
HIV risk and prevention behaviours among Prison Inmates in Georgia, 2015
Curatio International Foundation continues implementation of Bio-Behavioral Surveillance Surveys (BBS) among Key Affected Populations (KAP’s) with the aim to measure HIV prevalence among KAP’s, monitor risk behaviors among these groups and generate evidence for advocacy and policy-making. The current study…
Washington DC hosts workshop Immunization Costing: what have we learned, can we do better?
On May 17-18 EPIC Immunization Costing hosts workshop Immunization Costing: what have we learned, can we do better? in Washington DC. CIF executive director George Gotsadze and Business Develop ment unit director Ketevan Goguadze are invited to attend the event. George Gotsadze…
Bio-Behavioral Surveillance Survey among Men who have Sex with Men in two major cities of Georgia, 2015
Bio-Behavioral Surveillance Survey among Men who have Sex with Men in two major cities of Georgia, 2015 Curatio International Foundation continues implementation of Bio-Behavioral Surveillance Surveys (BBS) among Key Affected Populations (KAP’s) with the aim to measure HIV prevalence among…
EPIC Studies – Governments Finance, On Average, More Than 50 Percent Of Immunization Expenses, 2010–11
Journal Health Affairs publishes a new Article EPIC Studies: Governments Finance, On Average, More Than 50 Percent Of Immunization Expenses, 2010–11 coauthored by CIF team member Keti Goguadze. Abstract: Governments in resource-poor settings have traditionally relied on external donor support…
Bio-Behavioral Surveillance Survey among People Who Inject Drugs in 7 cities of Georgia, 2015
Curatio International Foundation continues implementation of Bio-Behavioral Surveillance Surveys (BBS) among Key Affected Populations (KAP’s) with the aim to measure HIV prevalence among KAP’s, monitor risk behaviors among these groups and generate evidence for advocacy and policy-making. The current study…
TB Community Systems Strengthening in Georgia
We are glad to announce that Curatio International Foundation has been selected to be Round 7 Challenge Facility for Civil Society (CFCS) grantee under the Stop TB partnership financial support. TB Community Systems Strengthening (TBCSS) project in Georgia aims –…
What can be done to improve treatment adherence among tuberculosis patients in Georgia: Looking through health systems lens
Curatio International Foundation has started implementation of new research project with the aim to provide a more in-depth understanding of the factors associated with loss to follow-up among TB patients. The project is funded under the Joint TDR/EURO Small Grants…
BioBehavior Surveillance Survey results were represented to the members of Parliament of Georgia
Curatio International Foundation together with BEMONI PUBLIC UNION (BPU) represented BioBehavior Surveillance Survey results to the Members of Parliament of Georgia. The study was conducted in seven major cities of Georgia (Tbilisi, Gori, Telavi, Zugdidi, Batumi, Kutaisi and Rustavi) with…
Announcing Winter Internship Program 2016
Curatio International Foundation invites Master and PhD international students to apply on Winter Internship Program. Through the program, students have the possibility to develop advanced research skills, meet leading experts in the field and become a coauthor of a scientific…
Regional High Level Dialogue ‘Road to Success’, Tbilisi, Georgia
On September 28-30, 2015 Regional High Level Dialogue took place in Tbilisi, Georgia on Successful Transition to Domestic Funding of HIV and TB Response in EECA. The 320 delegates from 31 countries have agreed to work together in creating and…
CIF study results on 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention
Findings of population size estimation study among Man who have Sex with Men (MSM) was presented to the 8th International Aids Association conference on HIV Pathogenesis, Treatment and Prevention in Vancouver, Canada in July, 2015. The study was conducted by…
Response to the “Final evaluation of GAVI support to Bosnia and Herzegovina”
Gavi, the Vaccine Alliance published response to the “Final evaluation of Gavi support to Bosnia and Herzegovina” conducted by Curatio International Foundation. Gavi assess the final evaluation and the given recommendations as an important document for the transition country program…
The drivers of facility-based immunization performance and costs. An application to Moldova
The drivers of facility-based immunization performance and costs. An application to Moldova. This is the article an International peer reviewed Journal Vaccine published, Co-authored by experts from the Curatio International Foundation. The study was a part of a multi-country coting and…
Awaiting the results of Prisoners’ Behavior Surveillance Survey (BSS)
Curatio International Foundation together with Infectious Diseases, AIDS and Clinical Immunology Research Center and Association Tanadgoma has conducted the Behavior Surveillance Survey with biomarker component. The study report of Behavior Surveillance Survey with biomarker component among 210 prisoners will be…
Costs of routine immunization services in Moldova: Findings of a facility-based costing study
An International peer reviewed Journal Vaccine, published an article Costs of routine immunization services in Moldova: Findings of a facility-based costing study. Authored by experts from the Curatio International Foundation. The study evaluates the total economic and unit costs of…
Analyses of Costs and Financing of the Routine Immunization Program and New Vaccine Introduction in the Republic of Moldova
In 2012-2014 Curatio International Foundation implemented the costing study that aimed to evaluate routine immunization program costs and financing as well as incremental costs and financing of a new vaccine introduction in the Republic of Moldova. The study was a…
Health Service Utilization for Mental, Behavioural and Emotional Problems among Conflict-Affected Population in Georgia
An International peer reviewed Journal PLOS One has published an article Health Service Utilization for Mental, Behavioral and Emotional Problems among Conflict-Affected Population in Georgia: A Cross-Sectional Study, authored by experts from the Curatio International Foundation, and the London School of…
Healthcare Utilization and Expenditures for Chronic and Acute Conditions in Georgia: Does benefit package design matter?
An International peer reviewed journal BMC Health Services Research publishes an article Healthcare utilization and expenditures for chronic and acute conditions in Georgia: Does benefit package design matter?, authored by experts from the Curatio International Foundation and London School of…
Curatio International Foundation Hosts Health Systems Global Secretariat in Tbilisi, Georgia
Curatio International Foundation Hosts Health Systems Global Secretariat in Tbilisi, Georgia. Health Systems Global (HSG) is the first international membership organization fully dedicated to promoting health systems research and related knowledge translation. HSG brings together researchers, policy-makers, funders, implementers, civil…
An Impact Evaluation of Medical Insurance for Poor in Georgia: Preliminary Results and Policy Implications
An International peer reviewed journal Health Policy and Planning has published an article An impact evaluation of medical insurance for poor in Georgia: preliminary results and policy implications, authored by Curatio International Foundation experts. The authors evaluated the impact of…
Policy Information Platform (PIP) Expert Consultation Meeting
Policy Information Platform (PIP) expert consultation was held in Istanbul on 29-30 January, 2015. At the meeting methodological issues, roadmap for the PIP implementation and evaluation approaches were discussed. CIF director George Gotsadze and Research Unit director Ivdity Chikovani participated…
Civil Society Forum organized by Country Coordination Mechanism
On January 29, at Courtyard Marriott Hotel was held a Civil Society Forum organized by Country Coordination Mechanism. The forum was part of country dialogue process regarding HIV/AIDS and Tuberculosis issues. During the meeting, civil society representatives shared results of…
CIF Publishes the Short Movie on 20 Years of Healthcare, 2014
Curatio International Foundation celebrates its 20 years anniversary and prepares short movie describing the history of health care reforms in Georgia after collapse of Soviet Union. The story is retailed directly by the people involved in the process and organizations…
CIF Publishes Anniversary Publication ’20 Years of Health Care, 2014
20 Years Anniversary brochure describes reforms that have significantly altered the landscape of health care in Georgia after collapse of the Soviet Union. The contents draw on important publications and oral narratives by those who have been initiators, implementers and witnesses to…
CIF Celebrated the 20th Anniversary
On September 19, Curatio International Foundation celebrated the 20th anniversary. Alongside with our work it turned 20 years from Georgian healthcare system reforming start up, after collapse of Soviet Union. CIF celebrated the anniversary together with the people who were…
Georgian Healthcare System Barometer: Experts’ Evaluations of Changes Taking Place in the Healthcare
Curatio International Foundation published results of the survey, representing expert evaluations of processes and changes taking place in the Georgian healthcare field. “Georgian Healthcare System Barometer” is based on evaluations of 98 experts and covers 6-month period – May-October, 2013….
BlogTalkRadio to Host CIF President
In October 2013, the BlogTalkRadio program focusing on health hosted Katevan Chkhatarashvili, the President of Curatio International Foundation. Ketevan talks about her public health experience and CIF’s role in transition of Georgia’s health sector. New Health Internet Radio with Global…
Winner of CIF Students Fellowship Program for 2013-2014
Ana Petriashvili became the winner of Curatio International Foundation Fellowship program for 2013- 2014. As the winner of the previous year, she too is the Master program student of International Public Health Department of Tbilisi State Medical University. Ana is…
The Forbes and Skoll World Forum to discuss HIV/AIDS Epidemic with Director of CIF
Which Region Has The Fastest-Growing HIV/AIDS Epidemic In The World?- Giorgi Gotsadze, Director of Curatio International Foundation was asked to discuss this issue with Forbes. The discussion precedes many health-related discussions to take place in September at the Clinton Global…
CIF Participation in the Private Sector in Health Symposium 2013
On July 6, Sydney, Australia hosted the Private Sector in Health Symposium 2013. Three rounds of parallel sessions covered diverse range of issues, such as regulation, influencing quality of care, health financing and a focus on equity. Mr. George Gotsadze,…
Article on Springer-Determinants of Risky Sexual Behavior Among Injecting Drug Users in Georgia
Curatio International Foundation has published the article on Springer, Aids and Behavior Section. The article covers the findings of CIF’s recent study on Injection risk practices and risky sexual behaviors among injection drug users (IDUs) and their sexual partners particularly…
Tobacco Use and Nicotine Dependence among Conflict-Affected Men in the Republic of Georgia
Article published on International Journal of Environmental Research and Public Health ISSN 1660-4601 by Bayard Roberts, Ivdity Chikovani, Nino Makhashvili, Vikram Patel and Martin McKee There is very little evidence globally on tobacco use and nicotine dependence among civilian populations…
HIV prevalence and risk behaviors among key populations- Study Findings Published
HIV prevalence and risk behaviors among key populations were studied in Georgia in 2012. The Biomarker Behavioral Surveillance Surveys (Bio-BSS) were carried out among People Who Inject Drugs (PWIDs) in six major cities, among Men who have Sex with Men…
Think Tanks Signed an Ethical and Quality Standards Document
On May 21, Curatio International Foundation together with fifteen Georgian Think Tanks signed an Ethical and Quality Standards Document. The document presents comprehensive standards to guide the work of Georgian think tanks and establishes principles to ensure that the think…
Hosting interns from international universities
In the framework of its internship program, during the first months of 2012 winter, Curatio International Foundation hosted two interns Lucia Callizo from Macalester college in Minnesota and Laura Covarrubias from Johns Hopkins Bloomberg School of Public Health. Interns made…
Hosting interns from international universities
In the framework of its internship program, during the first months of 2012 winter, Curatio International Foundation hosted two interns Lucia Callizo from Macalester college in Minnesota and Laura Covarrubias from Johns Hopkins Bloomberg School of Public Health. Interns made…
CIF’s contribution to Investing in Global Health and Development
Since the creation of the Global Fund, the world’s financing instrument in the fight against AIDS, TB and Malaria the role of private foundations has significantly increased in contributing to funding, participation in its governance and to the strategy of…
Assessing the Health Insurance for the Poor in Georgia
During the last two decades Government of Georgia initiated series of reforms introducing major changes in health financing policy and restructuring the health system to reverse the negative trends observed in equity, affordability and quality of essential health service for…
Curatio International Foundation Among Grant Management Solutions Awarded Partners
Grant Management Solutions 2 Awarded to MSH Partnership: Up to Five More Years of Technical Support to Grantees of the Global Fund to Fight Aids, Tuberculosis and Malaria Cambridge, MA, October 16, 2012– Management Sciences for Health (MSH) takes pride…
Fourth Wave Results of Pharmaceutical Study Published
Curatio International Foundation has completed a study exploring ‘Price, Affordability and Availability of Medicines in Georgia’. The study was divided into three stages and carried out in 2009-2001. The key aim of the study is to improve affordability and availability…
CIF Calls for participation in internship program for 2012-2013
Curatio International Foundation launches its internship program for 2012-2013 years and invites interns from around the world who are studying at masters or Ph.D. level and who are interested to have first-hand experience in the real-life setting and to contribute…
Curatio International Foundation revealed the winner of its annual scholarship program
Curatio International Foundation named Ana Kasradze as the winner of its annual scholarship program. Ana is the master’s program student at the Tbilisi State University, Department of International Public Health. Ana is actively involved in annual conferences and study visits…
Impact of global HIV/AIDS initiatives on health systems in Ukraine, Kyrgyzstan and Georgia
During January-July 2010 Curatio International Foundation in collaboration with London School of Hygiene and Tropical Medicine and other partners started exploring the effects of Global Fund HIV programmes on the roles of civil society organisations (CSOs) in Georgia, Kyrgyzstan and…
Submission of Applications for CIF Scholarship for Master Program Students near to deadline
The deadline for submission documentations for CIF web site is approaching to deadline, June 15. Master program students of Public Health Management or BA with focus on Public Health management from accredited universities in Georgia are encouraged to participate in…
Health care in Georgia is currently available for very rich and very poor
As the lead key informant to the policy brief on Medical Insurance for the Poor: impact on access and affordability of health services in Georgia says, the “health care in Georgia is currently affordable for very reach and very poor”….
Presentation of the findings of Assessment of Complex Non-Communicable Condition in Low Income Countries
Use of Multi-Method Rapid Evaluation to Assess Complex Non-Communicable Condition in Low Income Countries At Geneva Health Forum 2012 Curatio International Foundation presented a study preliminary findings which looks at evaluation of health systems performance in low-resource settings with regard…
Poster Presentation at Copenhagen 2012 Conference on HIV
Curatio International Foundation presented two posters at Copenhagen 2012 Conference- HIV in Europe. One of the posters presented results of Bio-Behavioural surveys among Injecting Drug Users in five cities of Georgia in 2008-2009 and specifically explored Low testing uptake and…
Contributing to publishing the paper: Circus monkeys or change agents? Civil society advocacy for HIV/AIDS in adverse policy environments
Curatio International Foundation has contributed to publishing the paper that explores the factors enabling and undermining civil society efforts to advocate for policy reforms relating to HIV/AIDS and illicit drugs in three countries in Eastern Europe and Central Asia: Georgia,…
National Center for Biotechnology Information published CIF’s scientific paper on Unsafe injection and sexual risk behavior among injecting drug users in Georgia
In August 2011, National Center for Biotechnology Information published CIF’s scientific paper on Unsafe injection and sexual risk behavior among injecting drug users in Georgia. The paper describes the prevalence and correlates of unsafe drug injecting and sexual behaviors among…
Findings of the pharmaceutical market study in 2009-2011 years
The key aim of the study is to improve affordability and availability of medicines for the population. Based on a three-year observation of pharmacies and different medicines in Georgia, Curatio International Foundation studied the practice in the pharmaceutical sector and…
Main findings of Catastrophic Health Expenditure Analysis in Georgia
The researcher of the Curatio International Foundation Natia Rukhadze presented the findings of Catastrophic Health Expenditure Analysis in Georgia at the “Seminar on Health Financing Reforms in Georgia” held in MoLHSA on October 26, 2011. The study was funded by…
Contribution to the development of National Health Care Strategy 2011-2015
Curatio International Foundation has contributed to the development of National Health Care Strategy 2011-2015: “Affordable and Quality Health Care”. Under the current strategy, the government intends to improve population health through a reduction of disease burden and mortality by 2015….
Catastrophic Health Expenditure Analysis in Georgia
On October 26, 2011 the researcher of the Curatio International Foundation Natia Rukhadze presented the findings of Catastrophic Health Expenditure Analysis in Georgia at the “Seminar on Health Financing Reforms in Georgia” held in MoLHSA. The study was funded by…
New web guide for using qualitative approaches to health systems research
By the end of summer 2011 Curatio International Foundation and London School of Hygiene and Tropical Medicine (LSHTM) developed the web resource which serves as a guideline for qualitative approaches in researching the health systems. Information of the web site…
CIF Study Published in BMC Magazine, The Role of Supportive Supervision on Immunization Program Outcome- a randomized filed trial from Georgia
The research article by CIF and international experts has been published in BMC International Health and Human Rights 2009. Article is a part of the supplement: The fallacy of coverage: uncovering disparities to improve immunization rates through evidence.The Canadian International…
CIF Calls for participation in internship program for 2011-2012
Curatio International Foundation launches its internship program for 2011-2012 years and invites interns from around the world who are studying at masters or Ph.D. level and who are interested to have first-hand experience in the real-life setting and to contribute…
Releasing results of Bio-behavioral surveillance survey among men having sex with men
Georgia is among the countries with low HIV/AIDS prevalence but with a high potential for the development of a widespread epidemic. However, over the past several years while transmission through injecting drug use is still the prevailing route for HIV…
Prices, Availability and Affordability of Medicines in Georgia-the New Study Report Endorsed
In December 2010 CIF wrapped up the second stage of the study exploring “Price, availability and affordability of medicines in Georgia”. The study aimed at increasing awareness of civil society and improved access to medicines for the population through strengthening…
Customer Satisfaction Research Report on Corporate Health Insurance Released
Curatio International Foundation releases report on Customer Satisfaction Research on Corporate Health Insurance. The report was supported by International Health Budget Monitoring Initiative of the Open Society Institute. The research prepared by the three experts of CIF (Marine Egutia, Natia…
Statement for the Media-The Study on Injected Drug Users Completed
Only 1/4 of Intravenous Drug Users are getting tested for HIV, putting their wife’s and girlfriends and the rest of the Georgian population at risk for a widening epidemic Curatio International Foundation, a Georgian think tank, says “motivating IDUS to…
National and subnational HIV/AIDS coordination: are global health initiatives closing the gap between intent and practice?
The research article prepared by the international experts and representatives of Curatio International Foundation was published in the international journal Globalization and Health web site. The article is available at US National Library of Medicine as well. The paper identifies…
Assessment of HIV/AIDS Surveillance System Pilot is Already Available
Curatio International Foundation has provided Assessment of HIV/AIDS surveillance system pilot. The operations research of HIV/AIDS surveillance pilot in Georgia was conducted in the framework of the project “Establishment of evidence-base for national HIV/AIDS program by strengthening the HIV/AIDS surveillance…
Findings of Behavior Surveillance Surveys (BSS) Endorsed
Curatio International Foundation in collaboration with Infectious Diseases, AIDS and Clinical Immunology Research Center and Public Union Bemoni and association Tanadgoma has carried out Behavior Surveillance Surveys with biomarker component among Injecting Drug Users (IDUs), Commercial Sex Workers (CSWs), and…
Internship at Curatio International Foundation is challenging for John Hopkins University Students
Throughout 2 weeks Curatio International Foundation will be hosting interns from Public Health School at John Hopkins University Sudit Ranade and Mollie Werlieb. Having the opportunity to undertake internship at Curatio International Foundation for the first ever time, students are…
Representatives of CIF Visit 2010 Global Health Information Forum
On January 28-30, Ms. Keti Goguadze and Ms. Ivdity Chikovani, Project Managers at Curatio International Foundation presented Spot lights of Georgia Health Information System Strengthening at 2010 Global Health Information Forum in Bangkok, Thailand. The Global Health Information Forum, hosted…
Training in Evidence Search-Acquisition
Through implementing the project: Strengthening Capacity of Civil Society for Promoting Research Evidence into Policy Development in Georgia, Curatio International Foundation aims to improve the skills of civil society representatives in developing policies that are a) evidence informed b) tailored…
Marking 15th Anniversary
Curatio International Foundation unveiled the most successful Public Health Management Student on its 15th Years anniversary marking event. On December 18, Curatio International Foundation granted the first student of the fellowship program established on the occasion of its 15th anniversary….
Hosting Club Discussion on Mental Health Policy
On December 16th Curatio International Foundation in partnership with UK-Georgia professional Network (UGPN) hosted policy club discussion on Mental Health policy issues. The meeting focused on development of recommendations for the improvement of the existing mental health service provision system…
Put Final Touches on the Training in Policy and Political Cycle
Advent of the far reaching reforms era in Georgia surged a great demand in cross-sectoral collaboration between the government, civil society and the media to ensure production of high quality evidence and increased demand for evidence informed public policy formulation….
A New Paradigm for Regulating Georgian Pharmaceutical Market
Curatio International Foundation in partnership with UK-Georgia professional Network (UGPN) conducted policy club discussion to increase awareness of the civil society organization regarding the Bill on Changes and Amendments to the Georgian Law on Drugs and Pharmaceutical Activities, which has…
CIF will Launch Fellowship for the Best Student of Healthcare/Public Health Management Faculty
Are you a successful last year bachelor student of healthcare/public health management? If yes, you are posed a golden opportunity to be granted the fellowship worth around 250 GEL monthly. From 2009 CIF will start awarding fellowships to the most…
CoReform Project and the Ministry of Health Mark the Completion of the First Stage of Trainings on ICPC2 Application
Today, July 24th the CIF under the auspices of the USAID funded CoReform project hosted the award ceremony dedicated to the completion of the first stage of the training course on Classifications for Hospital, Ambulatory and Laboratory interventions. 30 Master…
Georgia HIS Strategic Plan posted on Health Metrics Network website
HMN takes the initiative to publish HIS strategic plan produced by Curatio International Foundation on its website. The first baseline assessment of HIS was carried out in 2006 in East Georgia by Curatio International Foundation. It applied the HMN (Health…
First Stage of Training Course on Evidence Informed Policy Formulation Crowned
More than 20 executives from leading local and international non-governmental organizations in public policy formulation have successfully completed training workshop on ”Evidence Informed Policy”, on July 31-August 3, 2009 in Ureki, Georgia. The training workshop in Ureki was the first…
Findings of Behavior Surveillance Surveys (BSS) to be Endorsed Soon
Curatio International Foundation in collaboration with Infectious Diseases, AIDS and Clinical Immunology Research Center and Public Union Bemoni has carried out Behavior Surveillance Surveys with biomarker component among intravenous drug users (IDUs). The activity covered 5 Georgian regions -Tbilisi, Batumi,…
The Study on System-wide Effects of the Global Fund on Georgia’s Health Care Systems posted on GHIN website
The country case summary displayed on GHIN website was prepared as part of the academic consortium of the WHO Maximizing Positive Synergies between health systems and GHIs initiative. The study assesses the effects of the Global Fund funding on the…
Behavior Surveillance Survey among CSWs Covers Batumi and Tbilisi
Curatio International Foundation together with Infectious Diseases, AIDS and Clinical Immunology Research Center and Association Tanadgoma carried out Behavior Surveillance Survey with biomarker component among CSWs. The survey targeted the capital Tbilisi and regional city Batumi, sampling 280 individuals. The…
Sentinel Surveillance Method to Provide Reliable HIV/AIDS Statistical Data
In the framework of the Global Fund project “Establishment of evidence-base for national HIV/AIDS program by strengthening the HIV/AIDS surveillance system in the country” Curatio International Foundation has launched 2 sentinel surveillance sites at STI clinics. In March 2009 two…
Curatio International Foundation Contributes to Global HIV/AIDS Initiatives
Curatio International Foundation is a member of Global HIV/AIDS Initiatives Network (GHIN). As a part of the WHO Maximizing Positive Synergies publication “Interactions between Global Health Initiatives and Health Systems: Evidence from countries” Georgia Case study “System-wide effects of the…
Mr. George Gotsadze approved as a Permanent Member of the Global Fund TRP
By the decision of the Global Fund 19-th board meeting Mr.George Gotsadze, the Director of Curatio International Foundation was appointed as a Permanent Member of the Technical Review Panel (TRP). On May 5-6, 2009 at the Global Fund 19-th board…
The research article on Household Catastrophic Health Expenditure-evidence from Georgia and its policy implications published in BMC Health Services Research
On April 28,2009 the research article on Household Catastrophic Health Expenditure-evidence from Georgia and its policy implications was published on the online scientific journal BMC Health Services Research. The research undertaken by the highly qualified experts of Curatio International Foundation…
CIF Hosts workshop on Classifications for Hospital and Laboratory intervention
On March 5, 2009 the CIF, in the framework of USAID funded CoReform project organized the workshop on Classifications for Hospital and Laboratory intervention. On March 5, 2009 the workshop on Classifications for Hospital, Ambulatory and Laboratory interventions was held….
National Conference in the framework of the Global Fund funded project
On December 24, 2008 the first phase of the project “Establishment of evidence base for national HIV/AIDS program by strengthening of HIV/AIDS surveillance system in the country” was closed by the National Conference. The event highlighted crowning achievements of the…
CIF conducted a workshop to discuss HIV/AIDS Surveillance System Assessment results
Trainings on Mental Health Financing conducted with the financial support of Adam Smith Foundation end successfully. 25 representatives from Mental Health NGOs, Association of psychiatrics, psychiatric coalition, ombudsmen office and media enjoyed an opportunity of gaining sound understanding of conceptual…
CIF conducted a workshop in the framework of the project funded by the Global Fund
On March 31, 2008 a stakeholder workshop to introduce project goal and objectives, project components, main activities and project time-frame was held at the Hotel “Ambasadori”. The workshop was organized in the framework of the project “Establishment of evidence base…
The Global Fund Contracted Curatio International Foundation for Consulting Services
Curatio International Foundation was contracted for consulting services in the following three areas of operation and grant management support:diagnostic and remedial action planning in Global Fund grant countries facing implementation challenges;governance and oversight processes for Country Coordinating Mechanisms (CCMs); analysis…
CIF and MoLHSA conduct a workshop on Integrated Model and Strategic Plan for the Health Information system development in Georgia
On February 12, 2008 a stakeholder workshop to discuss an Integrated Model and Strategic Plan for the Health Information System development in Georgia was held at the Ministry of Labor Health and Social Affairs of Georgia (MoLHSA). The workshop was…
Regional Workshop on Rotavirus and Diarrheal Disease
On January 23-24, 2008 a Regional Workshop entitled “Rotavirus and Diarrheal Disease Control” was held in Tbilisi, Georgia. The workshop with 50 participants was sponsored by PATH and hosted by Curatio International Foundation. The workshop brought together representatives from Eastern…
National Avian Influenza Surveillance Guidelines
In 2007 two editions of the guideline were published within the framework of the project Strengthening Surveillance, IEC and Procurement Planning to address Avian Influenza in Georgia. The guidelines provide comprehensive recommendations addressed to the Georgian health system workers on…
Dr. George Gotsadze – CIF director and PATH board member presented “Hope for health in a weakened nation”
Dr. George Gotsadze, Director of the Curatio International Foundation was invited to present findings at PATH about the human health story of the dissolution of Soviet Union, the political and socio-economic transition that has challenged a weak health care system,…
Natia Rukhadze – CIF Researcher at the Global HIV/AIDS Initiatives Network (GHIN) international workshop in Dublin, Ireland
Natia Rukhadze, presented preliminary research findings of the study “Effects of GFATM on Georgia’s Health System Development” to the global stakeholders. The selected topic for the presentation was “Sustainability of GFATM program supported activities in Georgia”. The workshop, which was…
George Gotsadze – CIF director at fifth Global NHA symposium in Lund, Sweden
Dr. George Gotsadze has participated in the fifth Global NHA symposium hosted in Lund, Sweden. July 8-11, 2007 The Fifth Global NHA symposium was a two-day workshop, arranged by the Swedish Institute for Health Economics (IHE) in collaboration with the…
Yale University Announced Selection of Leading Georgian Public Health Expert, Ketevan Chkhatarashvili, as a 2007 Yale World Fellow Yale University announced the selection of the leading Georgian health policy advisor, Ketevan Chkhatarashvili, the preside
Yale University announced the selection of the leading Georgian health policy advisor, Ketevan Chkhatarashvili, the president of Curatio International Foundation, an organization which is a pioneer of health care reform throughout the Caucasus, Central Asia, Eastern Europe, and Africa, as…
Introductory Workshop on National Health Accounts in Azerbaijan
Dr. George Gotsadze, Director of Curatio International Foundation, traveled to Baku, Azerbaijan during May 16-18, 2007. Dr. Gotsadze was invited within the frame of Primary Health Care (PHC) Strengthening Project in Azerbaijan to contribute to the introductory workshop on National…
Protocol of the Policy Club on the Public Health Organizational Development
On February 23, 2007 the policy club has been organized within the framework of CoReform Project with the aim to discuss organizational arrangement of the Public Health at local level. It has brought together Deputy Ministers MOLHSA, representatives of Sector…
Health Research for a Responsive Healthcare System in Georgia
The project was funded by the Rockefeller Foundation and implemented by Curatio International Foundation (CIF) in collaboration with National Health Management Center of Georgia (NHMC), Tbilisi State Medical University, Department of Public Health and Management of Georgia (DPHM), London School…
Development of Hospital Master Plan for Georgia, 1998-1999
Development of Hospital Master Plan for Georgia project started in July 1998 and ended in January 1999. The project was funded by the World Bank through prime contract with Kaiser Permanente International (USA). Curatio International Foundation managed and administered all…
Hospital Financing in Georgia: Problems & Solutions, 1998-1999
The Hospital Financing in Georgia Problems & Solutions project was funded by the U.S. Agency for International Development (USAID) through prime contract with Abt. Associates Inc. The project aimed to evaluate financing of the hospital sector in Georgia in order…
Healthcare Reform in Georgia
Since the restoration of independence, reforming Georgia`s healthcare system was vital. The report looks at reforms implemented since early 90ies. It give a brief overview of heatlh situation in the Soviet union. Authors: David Gzirishvili, George Mataradze; 1998. Read the…
Final Report on Family Planning and Reproductive Health Assessment in Georgia
The overall goal of the assessment was to promote a development of the national policy on family planning in Georgia through provision of updated and reliable information about the family planning/reproductive health (FP/RH) in the country. A panel of experts…