External Reference Pricing Policy: A Possible Pharmaceutical Price Regulation Policy in Georgia
rmaceutical expenditure is a heavy financial burden and one of the factors of the impoverishment of the Georgian population. One of the leading factors contributing to the particularly high medicine expenditure in Georgia is a significant gap in pharmaceutical policy and regulations, which has created an environment conducive to both irrational use of medicines and uncontrolled growth of drug prices in the country
This document focuses on external reference pricing – one of the tools used to regulate pharmaceutical prices which makes medicines more accessible to the citizens brings their prices closer to those in neighboring countries and saves both a portion of the country’s budget spent on medicines and out-of-pocket pharmaceutical expenditure borne by the population.
The purpose of this document is to review the implications of external reference pricing (ERP) – one of the most widely used mechanisms for regulating medicine prices – for improving access to medicines and to summarize factors relevant for the ERP introduction so as to make policymakers avoid potential risks and dangers of the ERP introduction (if the latter is decided) by taking into account the existing international experience.
The document was developed by a group of authors – Lela Sulaberidze, Vakhtang Natsvlishvili, Nino Kotrikadze, Tsira Gvasalia, Konstantine Chachibaia. The evidence synthesis was prepared under the Evidence-based Policy and Practice Support Project implemented by Curatio International Foundation in partnership with the Knowledge to Policy Center (K2P) at the American University of Beirut.
Latest News
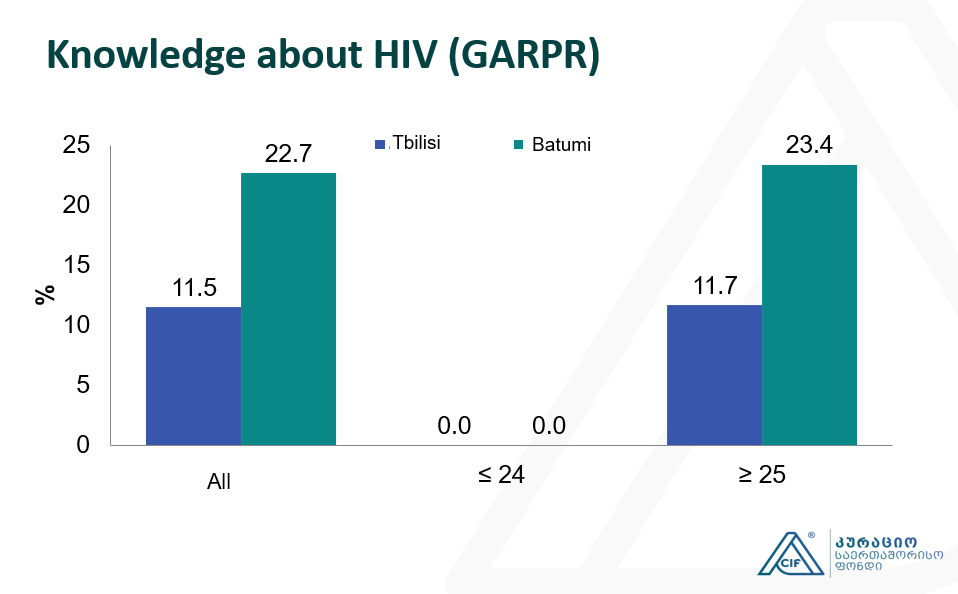
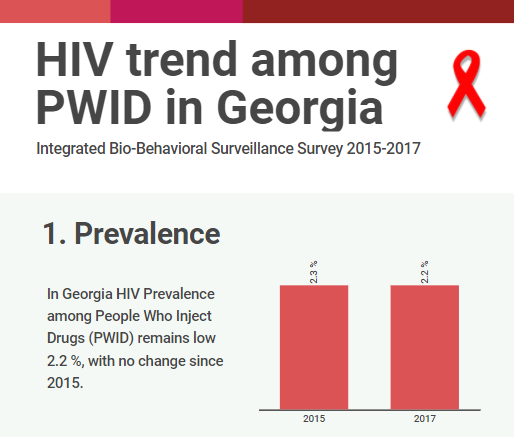
Integrated Bio-behavioral surveillance and population size estimation survey among Female Sex Workers in Tbilisi and Batumi, Georgia, in 2024
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Curatio International Foundation at Eighth Global Symposium on Health Systems Research (HSR2024)
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
“The Informatics and Data Science for Public Health: Sustainment Plan for Skilled Labor Force Development”
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Janina Stauke from the London School of Hygiene and Tropical Medicine shares her internship experience
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Georgia’s Journey to Integrating Rehabilitation Services into the Health System: Insights and Lessons
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Linked’s workshop on HPV vaccine introduction and scale up, held on July 11-12th, 2023
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Training program focusing on interdisciplinary evaluation of rehabilitation interventions and patient outcomes
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Unlocking Success Through Learning: Workshop on Strengthening HR Capacity and Performance Management in Immunization
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Promote evidence-based policies in the pharmaceutical sector by generating evidence and fostering civic engagement
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
CIF and the Results for Development / Accelerator combined their expertise to co-author an insightful blog, shedding light on Georgia’s commendable efforts to overcome limited data challenges and develop evidence-based policies for financing rehabilitation services
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Culminating event – Building Institutional Capacity for Health Policy and Systems Research and Delivery science (BIRD) in six WHO Regions
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Report on Phased (Stepwise) Plan for the Capability Development of the Priority Rehabilitation Services
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Sustaining Public Health Gains after Donor Transition: What can we learn about Georgia?
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Curatio International Foundation at Seventh Global Symposium on Health Systems Research (HSR2022)
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
New case study: Sustaining effective coverage with Opioid Substitution Therapy (OST) in Georgia in the context of transition from external assistance
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
“Strengthening Health Systems for Accessible Rehabilitation Services in Georgia” – Workshop
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
New Paper: A transdiagnostic psychosocial prevention-intervention service for young people in the Republic of Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Vaccine Procurement and Supply for the Expanded Program of Immunization in Kazakhstan
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Prevention of Addiction and Mental Health in Adolescents in Georgia (PAMAd) – Workshop
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Data Analysis and Synthesis Workshop – analyzing the implications of the structure of Georgia’s private healthcare market for quality and accessibility
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Paper: Soviet legacy is still pervasive in health policy and systems research in the post-Soviet states
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Article: How do participatory methods shape policy? Applying a realist approach to the formulation of a new tuberculosis policy in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Bundled Payment Methods: An Alternative Payment Method to Contain Healthcare Costs in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
HOW TO MAINTAIN ROUTINE IMMUNIZATION DURING COVID-19? EXPERIENCES FROM ARMENIA, GEORGIA, AND UZBEKISTAN
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Georgian Healthcare Barometer XIV Wave The analysis of financial stability and risks in healthcare
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
We are pleased to announce that The Sixth Global Symposium on Health Systems Research (HSR2020) has opened
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Discussing interim results of research project: Prevention of Addiction and Mental Health in Adolescents in Georgia (PAMAd)
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Effects of Pay for Performance on utilization and quality of care among Primary Health Care providers in Middle and High-Income countries
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
The first phase of the joint fellowship program of the Curatio International Foundation and the Knowledge to Policy Center (K2P) at the American University of Beirut has been successfully implemented
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
LNCT WEBINAR: Incremental Costs of Routine Immunization, Campaigns, and Outreach Services During COVID-19
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
LNCT WEBINAR: Designing Behavioural Strategies for Immunization in a Covid-19 Context
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Concentration and fragmentation: analyzing the implications of the structure of Georgia’s private healthcare market for quality and accessibility (ConFrag)
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
LNCT Webinar: Key Considerations for Integrating Immunization with Other Primary Health Care Services
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Project on “Technical Assistance Using Modern Technology for TB Prevention, Diagnosis, and Increased Quality Treatment” was closed
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Dialogue on Pharmaceutical pricing policies to improve the population’s access to pharmaceuticals in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
LNCT Webinar: Implementing a High Performing Immunization Program within the Context of National Health Insurance: What can we Learn from Thailand?
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Implementing new research: Prevention of Addiction and Mental Health in Adolescents in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Georgia’s introduction of the Hexavalent vaccine: Lessons on successful procurement and advocacy
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
CALL FOR MENTEES: PUBLICATION MENTORSHIP FOR FIRST-TIME WOMEN AUTHORS IN THE FIELD OF HPSR
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
A pilot of a new intervention launched to Improve adherence to TB treatment and its outcomes in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Workshop on using modern technology for TB prevention, diagnosis and increased quality treatment
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
K2P Mentorship Program on Building Institutional Capacity on Evidence Informed Policy Making
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Doing embedded development and research – reflections on the start of the Results4TB programme
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Introductory Meeting on the project ‘Embedding Rapid Reviews in Health Policy-Making’
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Building Institutional Capacity for HPSR and Delivery Science- CIF is Europe region HUB
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Inter-regional workshop in preparation for transitioning towards domestic financing in TB, HIV and Malaria programmes
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Memorandum of Cooperation between the Health and Social Issues Committee of the Parliament of Georgia and Curatio International Foundation
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
The civil society gathered for the fourth time to discuss healthcare system challenges in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Project: HIV risk behavior among Men who have Sex with Men – Bio-Behavioral Surveillance Survey and Population Size Estimation
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Big Pharma Greed and Artificial Prices – Knocking on Door to Limit Access to HIV Medicines in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Civil society is gathering for the third time to hold a discussion about the healthcare
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Webinar: Mapping and consensus of global competencies set for the field of HPSR: A progress update and HSG round table discussion
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Technical Assistance for evaluation of transition readiness and preparation of Transition and Sustainability Plan for Global Fund-supported programs in Tajikistan
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Technical Assistance for the preparation of Transition and Sustainability Plan for HIV program in Philippines
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Webinar: Integrating gender into health system strengthening in conflict and crisis-affected settings; what’s in our toolkit?
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Article: Barriers to mental health care utilization among internally displaced persons in the republic of Georgia: a rapid appraisal study
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Why Georgians second-guess their doctors – Deregulation has left Georgian medical care something many Georgians would rather avoid
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Webinar on The peer review process – what happens when you send your manuscript to a journal
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Webinar on Improving Quality of Care during Childbirth: Learnings and Next Steps from the BetterBirth Trial
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Integrated Bio-behavioral surveillance and population size estimation survey among Female Sex Workers in Tbilisi and Batumi, Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Applying a Health Policy and Systems Research lens to Human Resources for Health: Capacity building, leadership and politics
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Empowering civil society for engagement in and monitoring the decision making in health sector in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Curatio International Foundation presented BBS and PSE study findings at the Civil Society Forum
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Article: Barriers to delivering mental health services in Georgia with an economic and financial focus: informing policy and acting on evidence
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
The Interview on population size and Human Immunodeficiency Virus risk behaviors of People who Inject Drugs in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
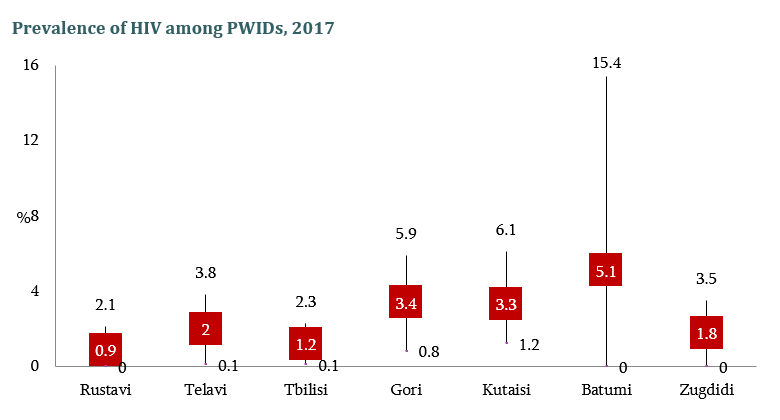
HIV risk and prevention behaviors among People Who Inject Drugs in seven cities of Georgia, 2017
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Conference paper: The Study of Barriers and Facilitators to Adherence to Treatment among Drug Resistant Tuberculosis Patients in Georgia to Inform Policy Decision
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Article: Human immunodeficiency virus prevalence and risk determinants among people who inject drugs in the Republic of Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Conference paper about realist evaluation: Informing policy, assessing its effects and understanding how it works for improved Tuberculosis management in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Georgian Healthcare and its Challenges: Healthcare Expert George Gotsadze will host the lecture
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
17 years in Curatio International Foundation: President Ketevan Chkhatarashvili to Leave Organization
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Georgian Solution for a Post-Soviet TB Program: Can Integration into Primary Health Care Improve TB Care?
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Article: Determinants analysis of outpatient service utilization in Georgia: can the approach help inform benefit package design?
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Designing and evaluating provider results-based financing for tuberculosis care in Georgia (RBF4TB)
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Barriers and Facilitators to Adherence to Treatment Among Drug Resistant TB Patients in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Eastern Europe and Central Asia Regional Sustainability and Transition Coordination Summit 20-21 October, 2016 Vilnius, Lithuania
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Washington DC hosts workshop Immunization Costing: what have we learned, can we do better?
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Bio-Behavioral Surveillance Survey among Men who have Sex with Men in two major cities of Georgia, 2015
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
EPIC Studies – Governments Finance, On Average, More Than 50 Percent Of Immunization Expenses, 2010–11
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Bio-Behavioral Surveillance Survey among People Who Inject Drugs in 7 cities of Georgia, 2015
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
What can be done to improve treatment adherence among tuberculosis patients in Georgia: Looking through health systems lens
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
BioBehavior Surveillance Survey results were represented to the members of Parliament of Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
CIF study results on 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
The drivers of facility-based immunization performance and costs. An application to Moldova
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Costs of routine immunization services in Moldova: Findings of a facility-based costing study
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Analyses of Costs and Financing of the Routine Immunization Program and New Vaccine Introduction in the Republic of Moldova
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Health Service Utilization for Mental, Behavioural and Emotional Problems among Conflict-Affected Population in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Healthcare Utilization and Expenditures for Chronic and Acute Conditions in Georgia: Does benefit package design matter?
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Curatio International Foundation Hosts Health Systems Global Secretariat in Tbilisi, Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
An Impact Evaluation of Medical Insurance for Poor in Georgia: Preliminary Results and Policy Implications
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Georgian Healthcare System Barometer: Experts’ Evaluations of Changes Taking Place in the Healthcare
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Article on Springer-Determinants of Risky Sexual Behavior Among Injecting Drug Users in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Tobacco Use and Nicotine Dependence among Conflict-Affected Men in the Republic of Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Curatio International Foundation revealed the winner of its annual scholarship program
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Impact of global HIV/AIDS initiatives on health systems in Ukraine, Kyrgyzstan and Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Submission of Applications for CIF Scholarship for Master Program Students near to deadline
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Presentation of the findings of Assessment of Complex Non-Communicable Condition in Low Income Countries
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Contributing to publishing the paper: Circus monkeys or change agents? Civil society advocacy for HIV/AIDS in adverse policy environments
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
National Center for Biotechnology Information published CIF’s scientific paper on Unsafe injection and sexual risk behavior among injecting drug users in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
CIF Study Published in BMC Magazine, The Role of Supportive Supervision on Immunization Program Outcome- a randomized filed trial from Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Releasing results of Bio-behavioral surveillance survey among men having sex with men
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Prices, Availability and Affordability of Medicines in Georgia-the New Study Report Endorsed
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
National and subnational HIV/AIDS coordination: are global health initiatives closing the gap between intent and practice?
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Internship at Curatio International Foundation is challenging for John Hopkins University Students
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
CIF will Launch Fellowship for the Best Student of Healthcare/Public Health Management Faculty
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
CoReform Project and the Ministry of Health Mark the Completion of the First Stage of Trainings on ICPC2 Application
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
The Study on System-wide Effects of the Global Fund on Georgia’s Health Care Systems posted on GHIN website
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
The research article on Household Catastrophic Health Expenditure-evidence from Georgia and its policy implications published in BMC Health Services Research
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
CIF and MoLHSA conduct a workshop on Integrated Model and Strategic Plan for the Health Information system development in Georgia
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Dr. George Gotsadze – CIF director and PATH board member presented “Hope for health in a weakened nation”
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Natia Rukhadze – CIF Researcher at the Global HIV/AIDS Initiatives Network (GHIN) international workshop in Dublin, Ireland
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით
Yale University Announced Selection of Leading Georgian Public Health Expert, Ketevan Chkhatarashvili, as a 2007 Yale World Fellow Yale University announced the selection of the leading Georgian health policy advisor, Ketevan Chkhatarashvili, the preside
დოკუმენტის მიზანია მედიკამენტების ფასების რეგულირების ერთერთი ფართოდ გავრცელებული მექანიზმის – გარე რეფერენტული ფასწარმოქმნის (გრფ) პოლიტიკის შედეგების მიმოხილვა მედიკამენტებზე ხელმისაწვდომობის გაუმჯობესების კუთხით